 In the latest attempt to look for biological correlates or predictors of mental illness, a paper in this month’s Archives of General Psychiatry shows that children with major depressive disorder (MDD) have thinner cortical layers than “healthy” children, or children with obsessive-compulsive disorder (OCD). Specifically, researchers performed brain MRI scans on 78 children with or without a diagnosis, and investigated seven specific areas of the cerebral cortex. Results showed four areas which were thinner in children with MDD than in healthy children, two which were thicker, and one that did not vary.
In the latest attempt to look for biological correlates or predictors of mental illness, a paper in this month’s Archives of General Psychiatry shows that children with major depressive disorder (MDD) have thinner cortical layers than “healthy” children, or children with obsessive-compulsive disorder (OCD). Specifically, researchers performed brain MRI scans on 78 children with or without a diagnosis, and investigated seven specific areas of the cerebral cortex. Results showed four areas which were thinner in children with MDD than in healthy children, two which were thicker, and one that did not vary.
These results add another small nugget of data to our (admittedly scant) understanding of mental illness—particularly in children, before the effects of years of continuous medication treatment. They also represent the bias towards imaging studies in psychiatry, whose findings—even if statistically significant—are not always that reliable or meaningful. (But I digress…)
An accompanying press release, however, was unrealistically enthusiastic. It suggested that this study “offers an exciting new way to identify more objective markers of psychiatric illness in children.” Indeed, the title of the paper itself (“Distinguishing between MDD and OCD in children by measuring regional cortical thickness”) might suggest a way to use this information in clinical practice right away. But it’s best not to jump to these conclusions just yet.
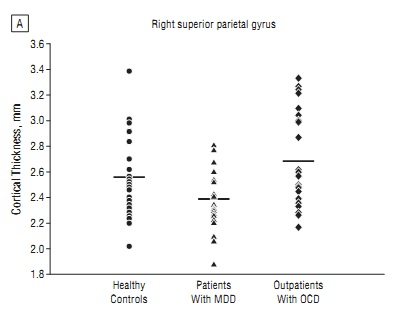 For one, there was tremendous variability in the data, as shown in the figure at left (click for larger view). While on average the children with MDD had a thinner right superior parietal gyrus (one of the cortical regions studied) than healthy children or children with OCD, no individual measurement was predictive of anything.
For one, there was tremendous variability in the data, as shown in the figure at left (click for larger view). While on average the children with MDD had a thinner right superior parietal gyrus (one of the cortical regions studied) than healthy children or children with OCD, no individual measurement was predictive of anything.
Second, the statement that we can “distinguish between depression and OCD” based on a brain scan reflects precisely the type of biological determinism and certainty (and hype?) that psychiatry has been striving for, but may never achieve (just look at the figure again). Lay readers—and, unfortunately, many clinicians—might read the headline and believe that “if we just order an MRI for Junior, we’ll be able to get the true diagnosis.” The positive predictive value of any test must be high enough to warrant its use in a larger population, and so far, the predictive value of most tests in psychiatry is poor.
 Third, there is no a priori reason why there should be a difference between the brains (or anything else, for that matter) of patients with depression and patients with OCD, when you consider the overlap between these—and other—psychiatric conditions. There are many shades of grey between “depression” and “OCD”: some depressed children will certainly have OCD-like traits, and vice versa. Treating the individual (and not necessarily the individual’s brain scan) is the best way to care for a person.
Third, there is no a priori reason why there should be a difference between the brains (or anything else, for that matter) of patients with depression and patients with OCD, when you consider the overlap between these—and other—psychiatric conditions. There are many shades of grey between “depression” and “OCD”: some depressed children will certainly have OCD-like traits, and vice versa. Treating the individual (and not necessarily the individual’s brain scan) is the best way to care for a person.
To be fair, the authors of the study, Erin Fallucca and David Rosenberg from Wayne State University in Detroit, do not state anywhere in their paper that this approach represents a “novel new diagnostic method” or make any other such sweeping claims about their findings. In fact, they write that the differences they observed “merit further investigation” and highlight the need to look “beyond the frontal-limbic circuit.” In other words, our current hypotheses about depression are not entirely supported by their findings (true), so we need to investigate further (also true). And this, admittedly, is how science should proceed.
However, the history of psychiatry is dotted with tantalizing neurobiological theories or findings which find their way into clinical practice before they’ve been fully proven, or even shown any great clinical relevance. Pertinent examples are the use of SPECT scans to diagnose ADHD, championed by Daniel Amen; quantitiative EEG to predict response to psychotropics; genotyping for metabolic enzymes; and the use of SSRIs to treat depression. (Wait, did I say that???)
 The quest to identify “biomarkers” of psychiatric illness may similarly lead us to believe we know more about a disease than we do. A biomarker is a biological feature (an endocrine or inflammatory measure, a genotype, a biochemical response to a particular intervention) that distinguishes a person with a condition from one without. They’re used throughout medicine for diagnosis, risk stratification and monitoring treatment response. A true biomarker for mental illness would represent a significant leap ahead in personalized treatment. Or would it? What if a person’s clinical presentation differs from what the marker predicts? “I’m sorry Mrs. Jones, but even though Katie compulsively washes her hands and counts to twelve hundreds of times a day, her right superior parietal gyrus is too thin for a diagnosis of OCD.”
The quest to identify “biomarkers” of psychiatric illness may similarly lead us to believe we know more about a disease than we do. A biomarker is a biological feature (an endocrine or inflammatory measure, a genotype, a biochemical response to a particular intervention) that distinguishes a person with a condition from one without. They’re used throughout medicine for diagnosis, risk stratification and monitoring treatment response. A true biomarker for mental illness would represent a significant leap ahead in personalized treatment. Or would it? What if a person’s clinical presentation differs from what the marker predicts? “I’m sorry Mrs. Jones, but even though Katie compulsively washes her hands and counts to twelve hundreds of times a day, her right superior parietal gyrus is too thin for a diagnosis of OCD.”
Other fields of medicine don’t experience this dilemma. If you have an elevated hsCRP and high LDL, even though you “feel fine,” you are still at elevated risk for cardiovascular disease and really ought to take preventive measures (exercise, diet, etc). (However, see this recent editorial in the BMJ about “who should define disease.”) But if your brain scan shows cortical thinning and you have no symptoms of depression, do you need to be treated? Are you even at risk?
 Some day (hopefully) these questions will be answered, as we gain a greater understanding of the biology of mental illness. But until then, let’s keep research and clinical practice separate until we know what we’re doing. Psychiatry doesn’t have to be like other fields of medicine. Patients suffer and come to us for help; let’s open our eyes and ears before sending them off to the scanner or the lab. In doing so, we might learn something important.
Some day (hopefully) these questions will be answered, as we gain a greater understanding of the biology of mental illness. But until then, let’s keep research and clinical practice separate until we know what we’re doing. Psychiatry doesn’t have to be like other fields of medicine. Patients suffer and come to us for help; let’s open our eyes and ears before sending them off to the scanner or the lab. In doing so, we might learn something important.



 Posted by stevebMD
Posted by stevebMD